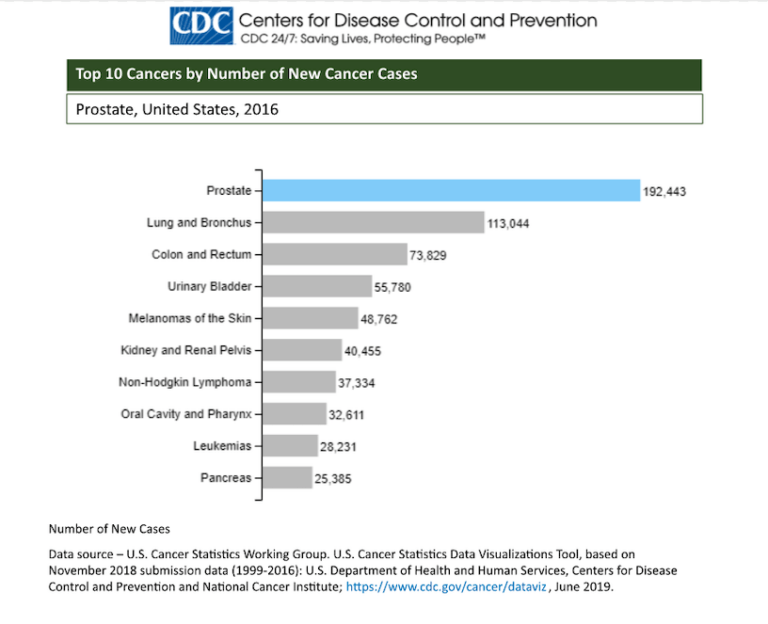
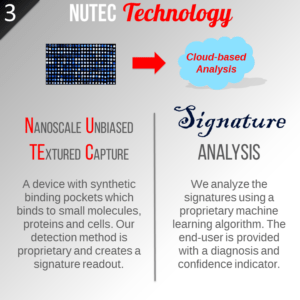
The GH215 study is a diagnostic platform that utilizes ‘NuTec signatures’ created by the unbiased binding of molecules from patient urine specimens as a means of detecting diseases in people. This platform has been successfully tested in various clinical proof of concept studies involving urine specimens from donors with and without various cancers and infections.
Reduce unnecessary biopsies, Reduce unwanted side effects, Reduce wasted time, Reduce costs, Improve Patient Care and Safety
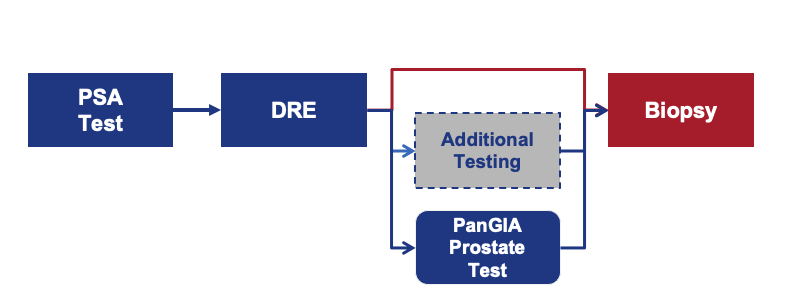
• Improved predictive profile over PSA / other screening tests
• Avoidance or warranted delay of invasive procedures
• Additional data points for identifying candidates for biopsies
• Improved outcomes from missing fewer cancers and reduced side effects
• Avoidance of unnecessary biopsies
• Avoidance of costs for side effects
Disclaimer: This test was evaluated, and its performance characteristics determined, by Genetics Institute of America. It has not been cleared or approved by the U.S. Food and Drug Administration. Such clearance or approval is generally not necessary. Genetics Institute of America is certified under the Clinical Laboratory Improvement Act of 1988 (CLIA) as qualified to perform high complexity testing.
Note: Please contact your insurance carrier for details regarding coverage for PanGIA Prostate.
References:
Sign up to receive monthly updates on new product announcements, press releases, promotions, and more.
Speak to one of our representatives today to learn more about our CAP and CLIA-accredited laboratory.
Copyright © 2019-2024 Genetics Institute of America. All Rights Reserved.